The honey bee gut is more than just a food processing chamber. It’s also home to hundreds of millions of microbes – some of which help the host, and some of which destroy it.
Has a round of antibiotics ever given you an upset stomach or an uncomfortable yeast infection? These are common side effects, but they’re not caused by the drugs directly; rather, the antibiotics kill our friendly bacteria along with the ones giving us grief. They don’t discriminate. The niche that was once filled with harmless and beneficial bacteria are invaded by selfish microbes, causing the unpleasant symptoms as an indirect side effect of antibiotics. Eventually the good microbes recolonize the gut, returning it to the normal, balanced microbial community – or microbiome – it once was.
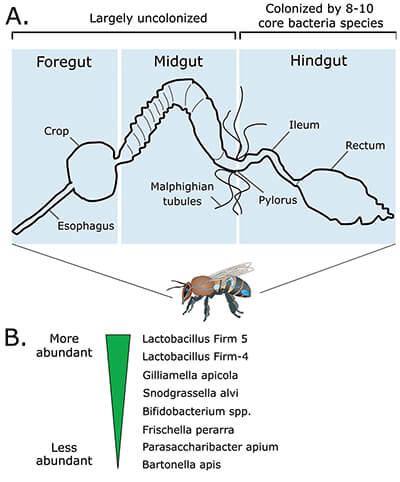
Honey bees are much the same. While estimates place the human gut microbiome at tens of trillions of microbial cells – maybe even 100 trillion, made up of up to 1,000 different species – honey bees are thought to have about one billion, composed of just 8–10 core species1,2 (Figure 1). Altogether, that’s still close, in sheer mass, to half the bee’s own brain, purely made up of bacteria and other microbes.
Similar to our own microbiome, these bacteria have important roles such as helping to fend off parasites and breaking down complex molecules into nutrients. Modern research now shows that the gut microbiome is more critical to honey bee health than we had previously thought.
The importance of getting to know this simple microbial ecosystem wasn’t lost on Nancy Moran, who is a long-standing aphid researcher currently running a bustling lab at the University of Texas at Austin. She was always interested in this idea of ‘symbiosis’ – particularly, the intimate relationship between a host and its microbes – and how it evolved. Moran saw a great opportunity for studying the symbiosis within honey bees and began pioneering the field of honey bee microbiome research at a time when it was underappreciated. But with her leading the way, researchers quickly realized it was a gold mine.
Moran was one of the first to begin studying how the gut microbiome established, and what having a healthy microbiome does for the host. What we learn about the honey bee gut microbiome will not only let us help our bees, it could also be applied to more complex systems, like that of our own gut. From 2003 onward, experiment after experiment painted the picture of the honey bee gut microbiome as being a surprisingly simple and stable community – a prime system for scientific study. And as it turns out, the relationship between these microbes and their host is ancient.
In 2010, Moran and other researchers found that eusocial Bombus and Apis species have a very similar core microbiome (i.e. they are colonized by many of the same bacterial species),1,3 whereas solitary bees have very different, sporadic communities. This suggests that there could be an evolutionary link between sociality and microbes, where these stable microbial communities developed because colonies – with their continual food-sharing and fecal-oral contact – offer a steady, reliable transmission system. The common ancestors to eusocial bee species probably began acquiring their bacteria well before these lineages diverged. With so long to coexist, these microbes are also remarkably well-adapted to live in harmony, and the best example of this is the co-dependence of two main bacterial players.
Gilliamella apicola and Snodgrassella alvi are two of the most ubiquitous honey bee gut inhabitants. Waldan Kwong, who was a student in Moran’s lab at the time (now a post-doctoral researcher at the University of British Columbia), wanted to understand the relationship between these two types of bacteria. “Snodgrassella forms a tight layer on the inner lining of the gut, and Gilliamella forms another layer on top of it,” Kwong explains. They almost always colonize the gut in this pattern, and their metabolism is extremely well-adapted for this specific situation. “The gut wall is more aerobic [oxygen-rich], whereas closer to the lumen is more anaerobic [oxygen-poor]. So Snodgrassella gets most of its energy through aerobic respiration, whereas Gilliamella gets most of its energy through anaerobic fermentation.” These tendencies to metabolize molecules differently is more than just an adaptation to the demands of living in slightly different local environments. It’s built right in to their DNA.
Gilliamella turns simple carbohydrates into energy through glycolysis – a process that occurs in virtually all living things. Normally, when glycolysis is finished, the leftover energy-containing molecules are fed into a second energy-production pathway: the Krebs cycle. But Gilliamella is missing the Krebs cycle genes.4 Instead, Gilliamella essentially hands the leftover molecules to Snodgrassella, which does have the necessary genes for the Krebs cycle, but is missing the genes for glycolysis. It’s a metabolic match made in heaven.
Taking a bigger picture view, Kwong was also interested in what other bacterial genes are beneficial for living in the gut. He and his colleagues systematically deleted each gene and looked for how that affected gut colonization. “In total, we found about ….


