What can be learned during varroa’s establishment in Australia?
I just moved to Australia for a year-long sabbatical. (Yes, it’s a major perk of being an academic researcher!) Given my current location, it probably isn’t surprising that my email in-box is filled with people asking what I’ve learned so far about “the varroa situation.”
If you don’t already know, until recently Australia was the last inhabited continent to avoid varroa. A truly impressive achievement given that varroa made its host switch from Apis cerana to Apis mellifera just a few thousand miles north in Japan, Hong Kong, and the Philippines in the 1950s and early 1960s, then spread through Asia, Africa, and Europe in the 1960s and 1970s, South America in 1971, North America in 1987, then neighboring New Zealand in 2000 (Traynor et al. 2020). Australia had been the last major holdout.
But in June 2022, varroa was detected in sentinel hives in the Newcastle area of New South Wales, just north of Sydney. Despite a $132 million ($84 million U.S.) eradication response plan from the Australian government [now abandoned, see “News and Events” in this issue], varroa infestations have now been detected as far north as Coffs Harbour and as far southwest as Balranald near the Victorian border (see Figure 1). In other words, it’s more or less certain this “varroa situation” isn’t going to be stopped.
Sad as it is that varroa is establishing in Australia, there are also opportunities to study its impact in real time. Such opportunities were rare during previous varroa invasions because beekeepers and scientists rarely had rigorous pre-invasion data in those locations. That isn’t true in Australia, which has heaps of great pre-invasion data. So, what will the consequences of varroa’s invasion be for colony health and loss rates? What about the tempo of miticide resistance, spillover of viruses into native wild pollinators, and impacts on pollination? These are the topics for the sixty-ninth Notes from the Lab, where I summarize “The final frontier: Ecological and evolutionary dynamics of a global parasite invasion,” written by Nadine Chapman and colleagues and published in the journal Biology Letters [2023].
The paper by Chapman and colleagues is an “ideas paper,” which means it doesn’t contain data. Instead, it contains lots of ideas for future research. These types of papers are useful because they discuss in detail the opportunities and challenges for research and monitoring, which can shape what is and isn’t done in terms of fundamental scientific studies (e.g., determining the genes governing host-parasite coevolution) and applied studies (e.g., predicting when chemical miticides will lose efficacy against varroa). In other words, an “ideas paper” on varroa is a great way to set the agenda that’s most likely to help scientists, beekeepers, farmers, and policymakers.
So, what do the authors think are the major opportunities for fundamental and applied research? Evolution, virology, and pollination ecology. We’ll tackle evolution first. The use of chemical miticides is well known to expose varroa to a selective pressure that can cause the mites to evolve resistance. For example, in the USA there’s widespread resistance to fluvalinate (Apistan) and coumaphos (Checkmite), and growing resistance to amitraz (Apivar) in some locations.
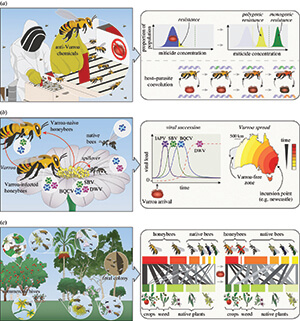
Resistance evolution is shown in Figure 2a, where a miticide is applied but isn’t lethal to 100% of the mite population. The mite population has a distribution of viability (blue), and the subsequent post-treatment mite population is formed from resistant individuals in the original population (yellow), which has polygenic resistance (i.e., multiple mite genes contributing to resistance). At higher levels of the miticide (dashed line), selection will effectively act on rare mutations at single genes and have a major impact on survival, leading to monogenic resistance (green).
Why is it important to know the details of monogenic vs. polygenic resistance? Perhaps surprisingly, little is actually known about the mode of action of miticides on varroa. By understanding the genetic basis of resistance (and therefore its mode of action), this knowledge can be used to develop other chemistries that are potentially more effective against varroa.
A second opportunity for evolutionary study comes from observing coevolutionary dynamics between varroa and honey bees in real time. It’s not just mites that will be evolving — bees will be evolving as well. As seen in the lower right side of Figure 2a, genetic adaptations can occur in mites, which in turn can facilitate adaptations to bees via natural selection or breeding. Understanding these changes is an opportunity to improve fundamental knowledge about host-parasite coevolution while also potentially informing breeding programs.
How about viruses? What do the authors think we can learn on that topic? When varroa arrived in New Zealand, virus succession followed a pattern similar to what’s shown in Figure 2b. Highly virulent viruses such as Israeli acute paralysis virus (IAPV) and sacbrood virus (SBV) rapidly increased in hives, followed by deformed wing virus (DWV) eventually becoming dominant.
Given what happened in New Zealand, a betting person might predict the same thing will happen in Australia. But there are also reasons to suspect something different will happen. Interestingly, there currently is no evidence from extensive testing that DWV is present in varroa-infested hives in Australia!
If DWV remains absent from Australia, it’s unclear if varroa will have the same impact on honey bees here compared to its impact in other countries. It’s widely assumed that the combination of varroa and DWV is what leads to a particularly lethal 1-2 punch for colonies. So, if DWV isn’t present, varroa infestations may end up being more benign. Alternatively, perhaps a different virus will take the place of DWV and team up with varroa to devastate colonies. Only research and monitoring over time will tell.
Regardless of whether DWV is introduced or not, the virus landscape for bees is going to change in Australia. This will impact honey bee colonies and native wild bees. Spillover of viruses from honey bees to native wild bees is currently a conservation concern, but which viruses are most important, which native wild bees are impacted, and what apiary densities and varroa infestation levels are necessary for spillover to occur is still poorly known. Again, research and monitoring throughout varroa’s establishment has great potential to shed new light on this topic.
How about pollination ecology? What do the authors think we can learn on that topic? If varroa’s introduction causes a population decline of feral honey bee colonies, as occurred in other parts of the world, the removal of unmanaged honey bee colonies from native and commercial ecosystems could change the pollination landscape (see Figure 2c).
In natural systems, reductions in honey bees could alter ….


