How can I help my bees get rid of all these mites?” A question that surely emerges in beekeepers’ heads when they open their colonies and see legions of hungry monsters crawling around. Fortunately, many weapons are available in the fight against Varroa destructor. Unfortunately, they do not completely eradicate the parasite. Worse, the most efficient synthetic miticides can also harm bees, accumulate in honey bee products, and miticide resistance is pervasive.1 In fact, the mite’s ability to adapt to compounds can be very swift: after only a few years of use, miticide-resistant mites take over colonies, rendering some treatments ineffective.2,3
Understanding how miticide resistance emerges and spreads in pest populations can help us improve our control strategies. One way to study this resistance is by using molecular techniques to look at areas of the genome that influence pests’ survival to treatments. For instance, genetic tools have been used by many researchers to understand how pyrethroid (tau-fluvalinate and flumethrin) resistance works in V. destructor. These compounds are among the most common class of miticides used against mites around the globe. They work by targeting the sodium channels (proteins in the parasite’s nerves), interfering with normal neuron signaling and eventually leading to death. Recently, studies revealed that if the sodium channel gene gets modified by a single mutation in exactly the right spot, they are no longer affected by pyrethroids.4–6 Mites that carry only one copy (or no copy) of this mutation are still susceptible, but mites that carry two copies inherited from both the maternal and paternal side are not. Miticide resistance is born.
Yet, as in all organisms, any mutations in the mite’s genome are rare and random. So why do we observe so many cases of resistance around the globe? How can such mutations become such a recurrent global problem? And most importantly, can we do a better job of mitigating it? My colleagues and I started investigating this topic two years ago. Back then, I was doing a PhD in Halle, Germany, where I focused on the interactions between honey bees and V. destructor.7 More particularly, we were interested in understanding how V. destructor mutations can spread within and between colonies and how this spread may fluctuate in time.8
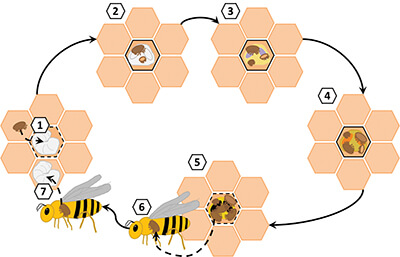
Reproduction in V. destructor
A peculiarity of the reproductive biology of V. destructor has a profound influence on how mutations spread in mite populations. The life cycle of V. destructor includes a series of reproductive and phoretic phases (Figure 1). Varroa reproduction starts when one or more mother mites (foundresses) enter a brood cell shortly before nurse bees seal it. The foundress first lays an unfertilized egg, which turns into a male. Then, she lays one fertilized egg every 30 hours, each of which turns into a female. Once the offspring are mature, they mate with each other, then all adult females (the mother and her daughters) emerge with the adult bee to begin the cycle anew. Thus, when only one foundress infests a cell, siblings have no choice but to mate with each other. In contrast, if more than one foundress infests the cell, offspring from different mothers can mate with each other. These two alternatives will produce genetically distinct progenies: the offspring produced by incestuous mating will be more inbred, and offspring produced from the crossing of more genetically distinct males and females will be outbred.
In most animals, inbreeding is bad for genetic diversity and leads to a higher prevalence of disease, but in mites, inbreeding actually helps them resist acaracides. In humans, many genetic disorders are more likely to occur after incestuous mating, such as haemophilia, microcephaly and albinism. These disorders are caused by so-called “recessive deleterious mutations,” which are only expressed when they are inherited from both parents. Since brothers and sisters have very similar genes, if they carry such deleterious mutations, the chance that their offspring will get two copies of the same one (and therefore suffer from a genetic disorder) are much higher than for unrelated parents. Resistance to miticides is inherited in a similar fashion. Just as inbreeding increases prevalence of rare genetic disorders, inbreeding also increases the chance of ….


