By now, readers of this column know that the honey bee colony is an assemblage of individuals, organized to a greater or lesser degree by genetic kinship, yet behaviorally specialized and integrated to optimize survival and reproduction of the group. Numerous times in this column we have drawn metaphors between organs, tissues, and behaviors in a metazoan organism, such as ourselves, and the corresponding behavioral castes in a honey bee colony. We can, for instance, see parallels in the decision-making process between a human brain deciding which car to buy and a honey bee swarm deciding which cavity to move into.
We have learned that new properties emerge out of the action of large groups when individuals in the group are free to make independent reactions to local stimuli. In this manner, for example, colony-level temperature regulation is the sum action of each individual bee shivering, clustering more tightly, or clustering more loosely in an attempt to find her “comfort zone.” The sum of everyones’ comfort zones adds up to the cluster surviving winter.
Similarly, the choice of nest cavity, the foraging for propolis, guarding behavior, and the allocation of foraging tasks to the oldest worker cohorts correspond to colony-level immune responses that limit the entry of nest enemies. And the chaotic construction of new comb by nectar-engorged bees results in the repeating parallel beeswax combs that provide square meters of texturally-rich substrate on which the daily drama of brood rearing, honey storage, and social interactions can play out.
All of this comprises a complex biological entity we call the honey bee superorganism, with biotic components (the bees) and abiotic (the cavity and beeswax combs), a reproductively autonomous Darwinian unit of selection, environmentally stable, proven and refined by natural selection to be an effective vehicle for transmitting down the generations the bundle of genes we call Apis mellifera.
The nest of a highly social insect can be so environmentally stable, both as a structural shelter and an oasis of optimum temperature and humidity, that it has been called “a factory in a fortress,1” the “factory” referring to the production of a worker force which in turn procures the energetic resources to sustain the colony’s reproduction, and the “fortress” in the case of the honey bee referring to the hollow cavities that scout bees seek out and appraise for their optimal volumes, insulative properties, and defensibility.
These hollows are usually in trees, range from 30-60 liters in volume, and have small entrances. Once bees occupy them the workers scrape away the dead soft wood, coat the sound wood and interior cavity walls with a propolis envelop, then proceed to build up to 3 m2 of parallel combs.2 This is the “skeleton,” if you will, of the honey bee superorganism, the substrate on which the bees through behavioral means regulate temperature and humidity for optimum living conditions. These cavities are then, by direct behavioral extension, rendered into ideal environments for insect adult life and immature development.
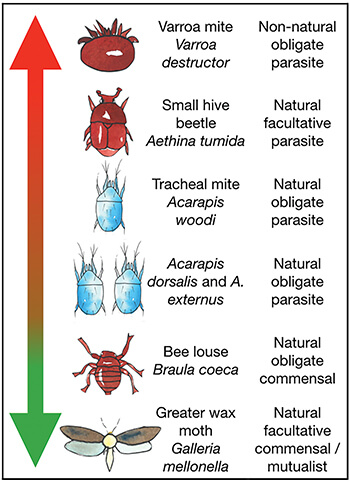
Other species think so too. In honey bees as with many other highly social insects, there are tenants in the house – whole bestiaries of organisms3 who live in the same nest, benefiting from the safe harborage, and making a living off the bees or nest detritus. Such close relationships between different species are called symbioses, and within that label the relationships can range from parasitic (one species benefits at the expense of the other), to commensal (one benefits, the other is not harmed), to mutualistic (each benefits). Additionally, these categories can be further categorized as obligate (the relationship is exclusive and necessary to at least one of the parties) to facultative (one or both can enter or leave the relationship as opportunity permits).
It is helpful to never forget that genes are inherently selfish and that natural selection rewards, without bias or impartiality, on the basis of one criterion only – whatever works. And “working” in this case means whatever heritable characters promote the survival and transmittal of the genes that code for them.
It’s easy to read cold-hearted selfishness in a parasite that takes the life of its host, but even the most benign of mutualists is playing by the same rules – only in this case, cooperation with its symbiotic partner has proven to be the best strategy for transmitting its genes. Once the calculus of gene transmittal tips in a different direction, the mutualist will be quick to adapt – on purely selfish grounds. So, when we talk about symbionts, it’s good to remember that these categories are, in the words of Hughes et al., “a continuum of costs and benefits with parasitism at one end and mutualism at the other.”4
Many authors have observed that large social insect colonies are like miniature ecosystems with layers of species and multi-trophic interactions similar to that observed in human scale terrestrial ecosystems. The same laws of ecology apply, so that, for example, whether it’s a social insect colony or a hedgerow in England, the number of species living in either ecosystem tends to increase with increasing size and age of the ecosystem. Large, long-lived, and stable systems tend to “collect” species over geologic time, many of which species may enter into symbiotic relationships with others, thus further enlarging and complicating the relationship webs.
In the ants and termites, we find the largest number, diversity, and complexity of nest symbionts – and for these very reasons: large size, long life, and stability. With virtually infinite space, the subterranean nests of ants and termites become diffuse and vast, a feature that encourages the evolution of secondary reproductives and large populations. Their nests include examples of sophisticated animal architecture, such as heat chimneys, that maintain favorable microclimates for insect nurseries and fungus gardens grown by the insects for food – among the most remarkable examples of symbiotic coevolution known in biology. Their highly diversified and numerous soldier castes provide waves of defensive protection, and in some species the reproductive pair live their post-dispersal lives protected in hardened mud bunkers deep in the nest.
In the end, these contrived habitats generate positive feedback loops that further perpetuate large populations, long colony life, and stability. These same conditions render complex social nests virtually predator free – yet another positive reinforcement, as life history theory predicts that low predation pressures encourage selection for long life.5 Is it any wonder that opportunistic species would evolve mechanisms to escape detection, to invade, to colonize, and to integrate into these attractive “fortress factories?”
And, reflecting on a recurring theme of these columns, yet again we see how features of the honey bee superorganism are recapitulated in the evolution of free-living organisms such as ourselves, so that by studying them we learn about us. For just as the honey bee superorganism has picked up symbionts in its long natural history, so too has our own lineage of Homo sapiens. There are “nest invaders” in our bodies, tenant species who run the gamut of parasitic (hookworms) to commensal (hair follicle mites) to mutualist (an array of microbiota essential to normal human physiology).6 And, whether in the honey bee superorganism or human organism, these symbionts have themselves shaped the evolution of their hosts. We could not be what we are without our symbionts.
The convergences between organisms and superorganisms are instructive for understanding…


